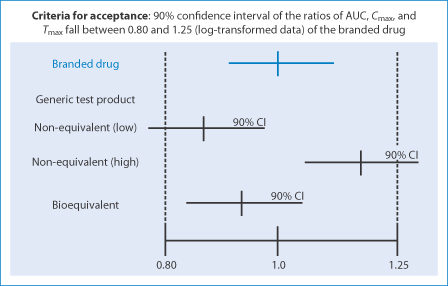
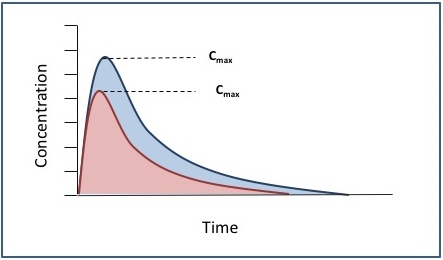
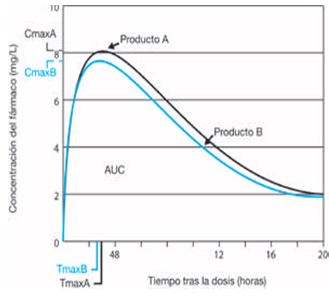
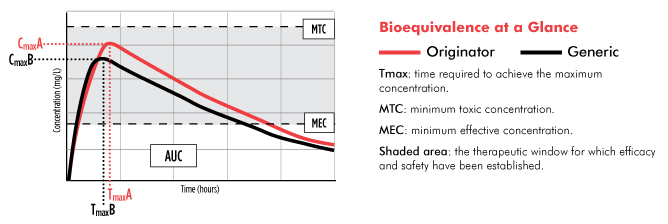
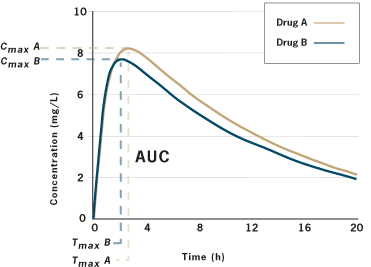
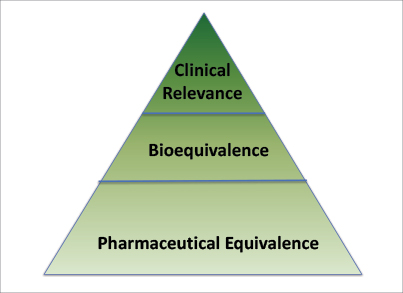
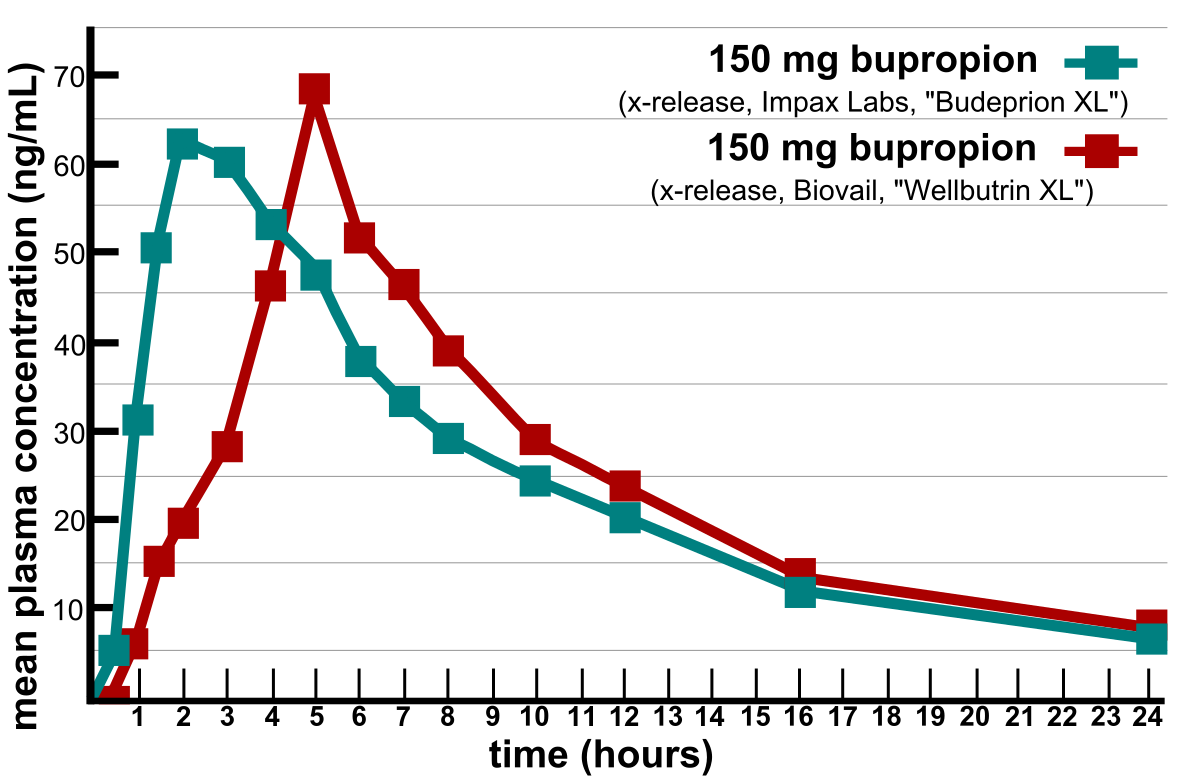Generics Substitution, Bioequivalence Standards, and International Oversight: Complex Issues Facing the FDA: Trends in Pharmacological Sciences
![PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/25e18dc07f478ecfade16b741d6768a6cb3da9b3/3-Table1-1.png)
PDF] Bioequivalence study of 30 mg pioglitazone tablets in Thai healthy volunteers. | Semantic Scholar
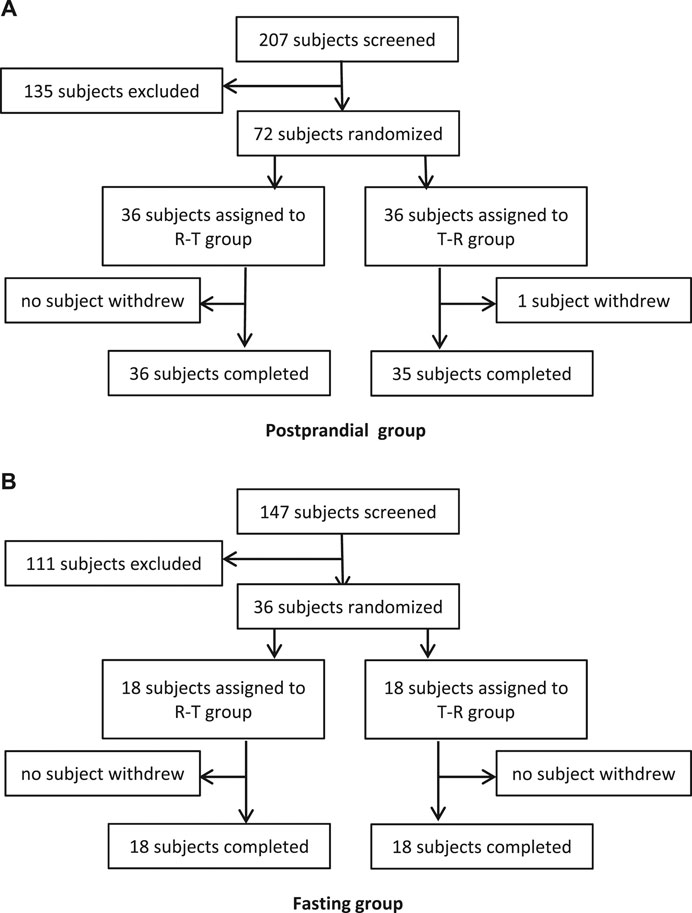
Frontiers | Pharmacokinetics and Bioequivalence of Rasagiline Tablets in Chinese Healthy Subjects Under Fasting and Fed Conditions: An Open, Randomized, Single-Dose, Double-Cycle, Two-Sequence, Crossover Trial
Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial | PLOS Medicine
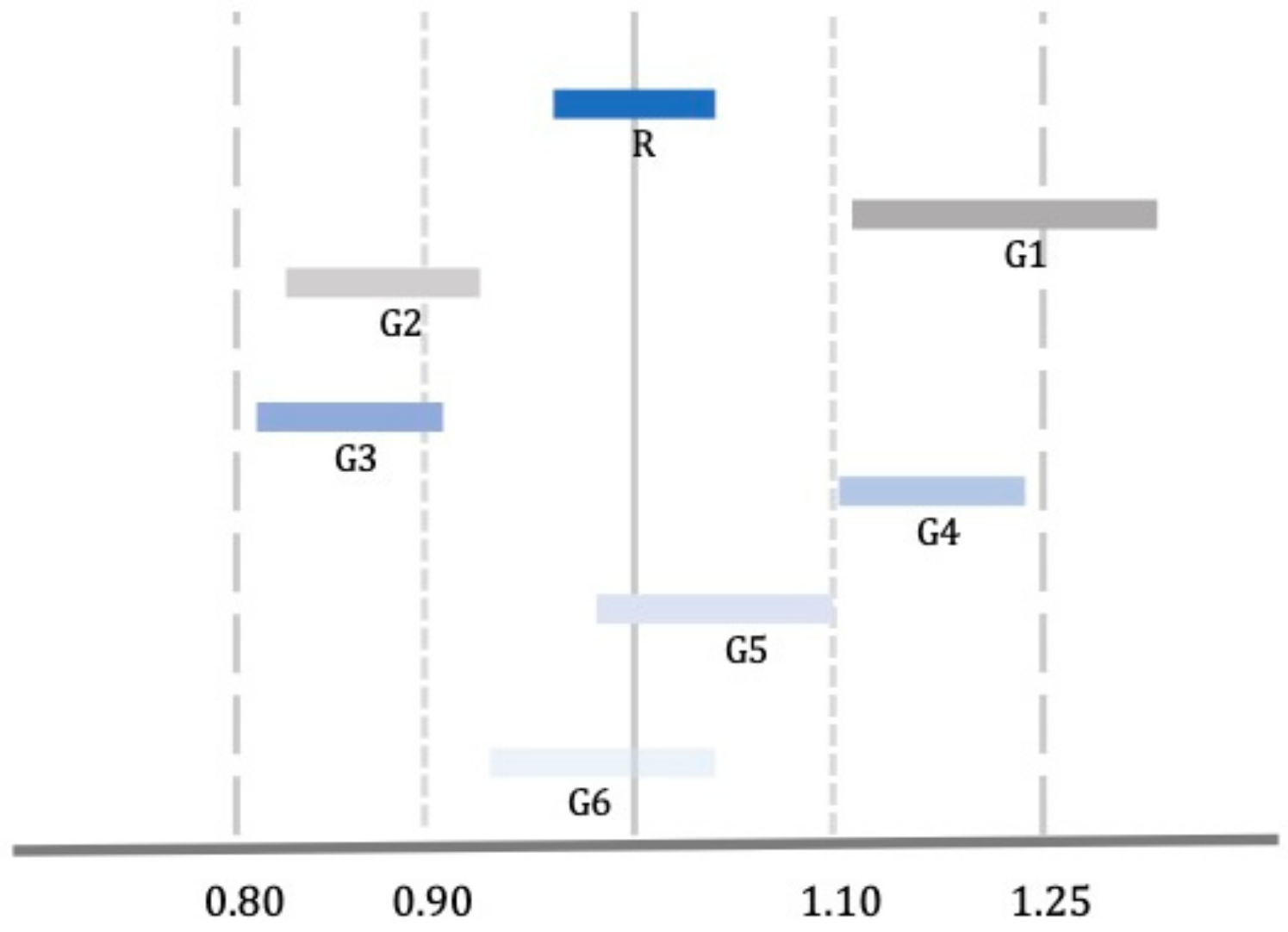
Healthcare | Free Full-Text | Bioequivalence, Drugs with Narrow Therapeutic Index and the Phenomenon of Biocreep: A Critical Analysis of the System for Generic Substitution

Switching among branded and generic medication products during ongoing treatment of psychiatric illness | BMJ Innovations